Data Set Characteristics Version 1
Ames Mutagenicity
| positive compounds |
3767
|
| negative compounds |
3323
|
| total |
7090
|
The classes of positive and negative Ames Test results are balanced.
Ames Test In the Ames test, frame-shift mutations or base-pair substitutions may be detected by exposure of histidine-dependent strains of Salmonella typhimurium to a test compound. When these strains are exposed to a mutagen, reverse mutations to the original histidine-independent form enable bacterial colony growth on a medium deficient in histidine ("revertants"). Since many chemicals interact with genetic material only after metabolic activation by enzyme systems not available in the bacterial cell, the test compound is additionally examined in the presence of a mammalian metabolizing system (with S9 mix). A compound is classified
positive if it significantly induces revertant colony growth at least in one strain, either in the presence or absence of S9 mix. A compound is labeled
negative if it does not induce revertant colony growth in any strain tested, both in the presence and absence of S9 mix.
Molecular Weight
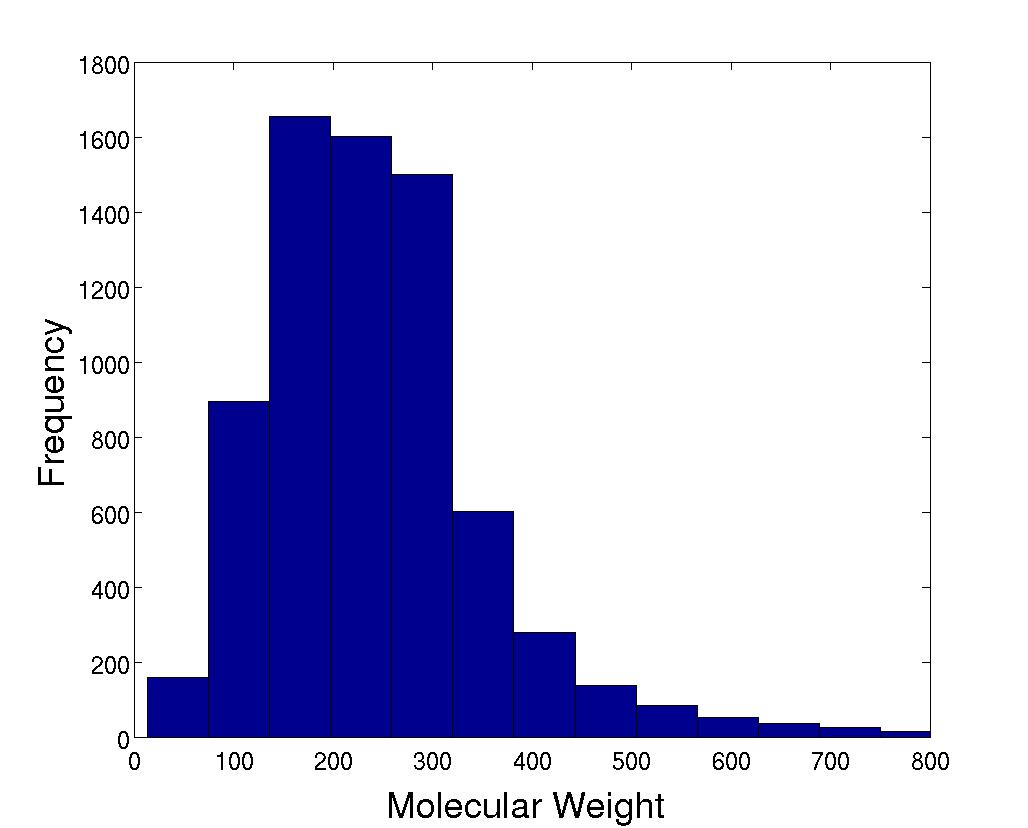 |
The molecular weight averages 248. |
Data Sources
The data was collected from public sources:
| Source |
no of compounds |
reference |
|
CCRIS
|
2614
|
[1]
|
|
EPA1
|
2527
|
[2]
|
|
EPA2
|
151
|
[3]
|
|
EPA3
|
490
|
[4]
|
|
VITIC
|
1278
|
[5]
|
|
GENETOX
|
30
|
[6]
|
[1] Chemical Carcinogenesis Research Information System. http://www.cancerinformatics.org.uk/matrix/CCRIS.htm
[2] Kazius J et al. Derivation and Validation of Toxicophores for Mutagenicity Prediction. J. Med. Chem. 2005, 48, 312-320
[3] C. Helma et al. Data Mining and Machine Learning Techniques for the Identification of Mutagenicity Inducing Substructures and Structure Activity Relationships of Noncongeneric Compounds. J. Chem. Inf. Comput. Sci. 2004, 44, 1402-1411
[4] J. Feng et al. Predictive Toxicology: Benchmarking Molecular Descriptors and Statistical Methods. J. Chem. Inf. Comput. Sci. 2003, 43, 1463-1470
[5] PN Judsom et al. Towards the Creation of an International Toxicology Information Centre. Toxicology 213(1-2):117-28, 2005
[6] Genetic Toxicity, Reproductive and Development Toxicity, and Carcinogenicity Database. http://www.fda.gov/Cder/Offices/OPS_IO/genrepcar.htm


